
What characteristics do cathode rays have? 2 marksĪns.

They also discovered that the quantity of voltage, kind of gas, and constituent ratios did not affect the natural, physical, and behavioral features of electrons. The theory helped future physicists in understanding the modern structure of an atom. Thomson derived a conclusion after the experiment that the rays that moved from the cation to the anion were negative. The phosphorescent substance was put in such a way that when the rays strike it, little sparks of light appear, allowing the stream of rays to be detected.The cathode rays begin deflecting and repelling from the dipole and moving towards the anode as a result.A dipole is used to detect and measure the beam that was created.A closed-loop circuit propagates electric current.Ionization of partial air inside the terminal due to high voltage causes gas to become the conductor.The equipment is set up so that the terminals have a high voltage and the internal pressure is decreased by eliminating air from the tube.The cathode ray was redirected away from the negatively charged plate and towards the positively charged plate. Thomson used two opposingly charged electric plates around the cathode ray to test the particles' characteristics. Thomson, a scientist, began working with cathode ray tubes in the late 1800s. Cathode rays are not visible, but they were first identified in early vacuum tubes when they impacted the tube's glass wall, energizing the atoms and causing them to emit light-a glow known as fluorescence.In simple words, rays traveling from the negative end to the positive end of an electrode within a vacuum are known as Cathode Rays.A focused beam of electrons is deflected by magnetic or electric fields in cathode ray tubes, in order to produce the image in a traditional television set (CRTs).Ĭathode Rays used to produce images in traditional Television The constituents of cathode rays were the first to be found. When a voltage is given to an evacuated glass tube with two electrodes, electrons emitted from the cathode cause the glass opposite the negative electrode to glow. In vacuum tubes, cathode rays (electron beams or e-beams) are electron streams.
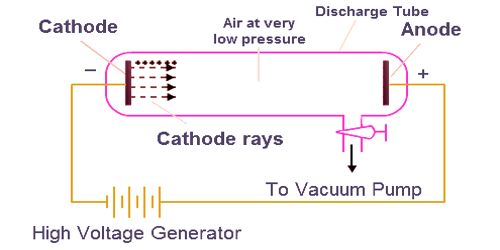
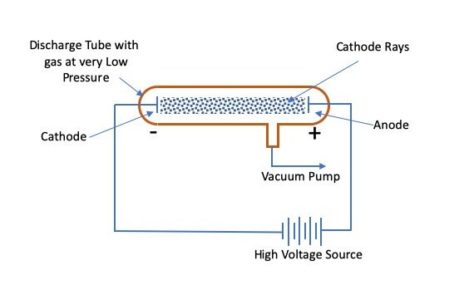
When the electron beam hits the phosphor-coated screen, it creates a small, brilliant visible spot on the fluorescent screen.


 0 kommentar(er)
0 kommentar(er)
